Investigation of the full reversal of selectivity in the reaction of aniline with 1,3-dichloro-1,3-bis(dimethylamino)vinamidinium salts†
Abstract
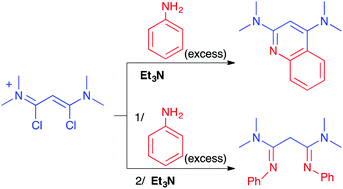
The addition of aniline, even in excess, to a solution of 1,3-dichloro-1,3-bis(dimethylamino)vinamidinium salt in the presence of triethylamine invariably leads to the formation of 2,4-bis(dimethylamino)quinoline. Conversely, delaying the addition of the base leads to the formation of 1,3-dimethylamino-N,N′-diphenylpropane-1,3-diimine, even when only a substoichiometric amount of aniline was used. A DFT study led to the consideration of factors that accounted for this reversal of reactivity. The role of a secondary orbital interaction in key transition states, as well as the role of solvent, is discussed.


 Please wait while we load your content...
Please wait while we load your content...