Solid-state photochromic behavior of pyrazolone 4-phenylthiosemicarbazones†
Abstract
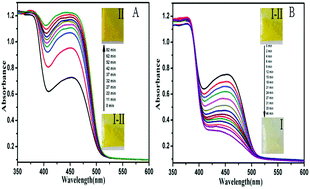
Solid-state photochromic materials have attracted much more attention due to their actual potential applications in photoactive devices. Here, three new compounds (1-phenyl-3-methyl-4-(3-fluoro/chloro/bromobenzal)-5-hydroxypyrazole 4-phenylthiosemicarbazones) were synthesized, which exhibited reversible solid state photochromic properties upon UV/Vis light irradiation. Their photochromic properties, first-order kinetics and fatigue resistance were investigated by time-dependent UV-Vis absorption spectroscopy. The results showed that their fatigue resistance and addressability were enhanced with the increase in the electron-withdrawing ability of the substituents on the phenyl group at the 4-position of the pyrazolone ring. Thus, 1-phenyl-3-methyl-4-(3-fluorobenzal)-5-hydroxypyrazole 4-phenylthiosemicarbazone exhibited good photochromic properties and high fatigue resistance. Based on single crystal X-ray diffraction and FT-IR analyses, the photochromic mechanism involved intra/intermolecular proton transfer.


 Please wait while we load your content...
Please wait while we load your content...