Cyanation of aromatic/vinylic boronic acids with α-cyanoacetates†
Abstract
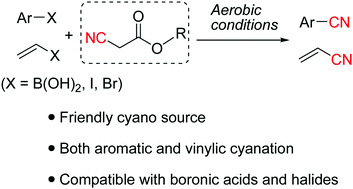
A friendly protocol is reported to achieve cyanation of aromatic and vinylic boronic acids using nontoxic and readily available α-cyanoacetates as a cyano source under aerobic conditions. Many aryl/vinyl boronic acids (as well as some iodides and bromides) are amenable substrates to give aryl nitriles and acrylonitriles. This cyanation method provides a safe and operationally convenient alternative to traditional ones requiring toxic cyanide salts.


 Please wait while we load your content...
Please wait while we load your content...