Synthesis and in vitro phototoxicity of novel π-extension derivatives of chlorin e6†
Abstract
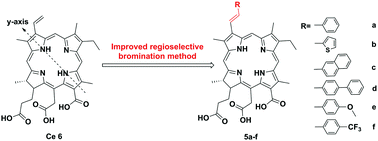
Six novel E-32-aryl substituted chlorins (5a–5f) were synthesized via improved regioselective bromination and Suzuki–Miyaura cross-coupling reactions with chlorin e6 (1) as a starting material. All the π-extension photosensitizers 5a–5f exhibited significant bathochromic-shifts (ca. 10 nm) in the far red part of the spectrum called the phototherapeutic window and decreased photobleaching under irradiation of 660 nm light. Moreover, 5a–5f possessed higher phototoxicity in vitro against HepG2 cells (IC50 = 0.73 ± 0.04, 2.33 ± 0.22, 0.78 ± 0.05, 0.50 ± 0.04, 2.25 ± 0.16 and 0.65 ± 0.11 μM, respectively) compared with chlorin e6 (IC50 = 28.9 ± 1.2 μM). The enhanced cellular uptake and reactive oxygen species (ROS) production of modified chlorins were found to be responsible for their increased in vitro phototoxicity.


 Please wait while we load your content...
Please wait while we load your content...