The regioselectivity in the platinum-catalyzed domino reaction to access alkynylated indoles: a theoretical study†
Abstract
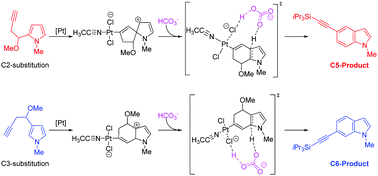
The reaction mechanisms of the platinum-catalyzed domino reaction to access C5- or C6-alkynylated indoles have been theoretically investigated by employing density functional theory (DFT) calculations. Our calculation results are in agreement with the experimental facts that C5- and C6-alkynylated indoles are the major products obtained by using C2- and C3-substituted pyrroles as the corresponding substrates, respectively. Based on our mechanistic study, the HCO3− additive used in the experiment is proven to play an important role in promoting deprotonation and aromatization steps. Through investigating the natural bond orbital (NBO) charges of the regioselectivity-determining transition states, one can find that the substrate-controlled regioselectivity is dominated by the electronic effects.


 Please wait while we load your content...
Please wait while we load your content...