Controlled growth of metallic copper nanoparticles
Abstract
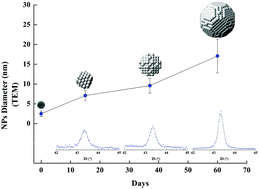
We present a comprehensive characterization of Cu nanoparticles (NPs) synthesized by a polyol method, combining ethylene glycol, copper chloride, polyvinylpyrrolidone, sodium citrate and ascorbic acid. This simple and low cost synthesis results in a colloid containing nearly-monodispersed metallic NPs. Our investigation enabled two important stages to be distinguished during the growth reaction: (1) the first, revealed by in situ time resolved dispersive X-ray absorption spectroscopy (XAS), corresponds to a fast reduction/nucleation and early growth of metallic NPs, later corroborated by ex situ XAS measurements. The resulting Cu nanoparticles have a mean diameter of 1.5 ± 0.8 nm, as determined by TEM; (2) the second stage, monitored by TEM, HRTEM and XRD measurements, corresponds to a slow aggregation growth where the mean volume grows linearly with time with a rate of 2.1 ± 0.3 nm3 per day – proceeding while the NPs are kept in the colloidal solution. Such slow growth rate allows the aging time to be used for tuning the NP size; nevertheless, we show that size dispersion also increases with time following a similar rate.


 Please wait while we load your content...
Please wait while we load your content...