Metal-free denitrative arylation of β-nitrostyrenes using benzoyl peroxide: an easy access to trans-stilbenes†
Abstract
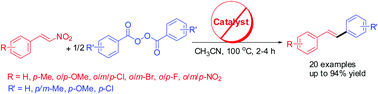
A simple, novel and stereoselective synthesis of trans-stilbenes has been described using denitrative arylation of β-nitrostyrenes in the presence of benzoyl peroxide under metal-free conditions. The reaction is assumed to involve homolytic cleavage of benzoyl peroxide followed by decarboxylation to generate a phenyl radical, which brings about ipso-substitution of the nitro group of nitrostyrenes to afford trans-stilbenes.


 Please wait while we load your content...
Please wait while we load your content...