Mathematical equations combined with the MHE-GC method to study desorption kinetics of contaminants from food-package paper to air†
Abstract
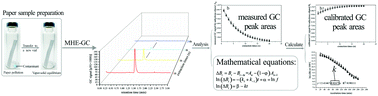
In this study, a technique based on multiple headspace extraction gas chromatography (MHE-GC) was applied to determine the transfer kinetics of contaminants from paper-based packaging materials to air because of its sensitivity and safety. Mathematical equations were derived in order to calculate the desorption reaction rate constant of a contaminant. This procedure was applied to five contaminants: o-xylene, ethanol absolute, n-dodecane, diphenyl oxide, and diallyl-o-phthalate. The results indicated that the desorption reaction rate constant increased with temperature. The vapor pressure and chemical structure of the contaminants also affected the desorption reaction rate constant. Temperature had a predominant effect on the desorbing kinetics of the contaminants. The equilibrium times of desorption decreased as the temperature increased; for example, the equilibrium times of o-xylene were ≥4, 4, and 1 h at 40, 70, and 100 °C, respectively.


 Please wait while we load your content...
Please wait while we load your content...