A novel family of homoleptic copper(i) complexes featuring disubstituted cyanamides: a combined synthetic, structural, and theoretical study†
Abstract
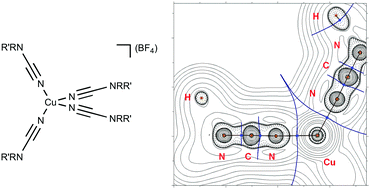
The homoleptic copper(I) complexes [Cu(NCNRR′)4](BF4) (R/R′ = Me/Me 1, Et/Et 2, C5H103, C4H8O 4, C4H85, C3H6C6H46, CH2Ph/CH2Ph 7, Me/Ph 8) featuring disubstituted cyanamides were obtained in excellent (92–97%) yields by the reaction of [Cu(NCMe)4](BF4) and 4 equivalents of NCNRR′. Complexes 1–8 were characterized by atomic absorption spectrometry (Cu%), high resolution ESI+-MS, molar conductivities, TG/DTA, and 1H, 13C{1H} NMR, FTIR spectroscopic techniques, and also by single-crystal X-ray diffraction (1, 3, and 4). Results of DFT calculations and X-ray structure determinations reveal that equilibrium geometries of [Cu(NCMe)4]+ and [Cu(NCNMe2)4]+ in the gas phase are normal tetrahedral (Td) and significantly distorted, respectively. Effects of crystal packing influence the values of the Cu–N–C angles in [Cu(NCNRR′)4]+, which points out to the noticeable contribution of the heterocumulene mesomeric form for the dialkylcyanamide copper(I) complexes. The QTAIM and NBO analyses indicate that relatively weak Cu–N contacts (15–31 kcal mol−1) in both cases exhibit single bond character and clearly polarized toward the N atom (by 91–95%). The CDA shows that the {M} ← L σ-donation substantially prevails over the {M} → L π-back-donation in both [Cu(NCMe)4]+ and [Cu(NCNMe2)4]+. The orbital, charge, and vibrational frequency arguments as well as inspection of the FTIR data suggest that the electrophilic activation of the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C group in homoleptic nitrile and dialkylcyanamide copper(I) complexes is similar, and the different behavior of nitriles and cyanamides in the 1,3-dipolar cycloaddition of ketonitrones is mainly due to the difference in the atomic charges.
C group in homoleptic nitrile and dialkylcyanamide copper(I) complexes is similar, and the different behavior of nitriles and cyanamides in the 1,3-dipolar cycloaddition of ketonitrones is mainly due to the difference in the atomic charges.


 Please wait while we load your content...
Please wait while we load your content...