Nanosized manganese oxide/holmium oxide: a new composite for water oxidation†
Abstract
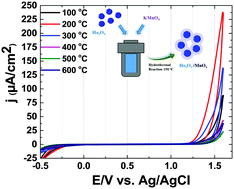
Herein, holmium oxide as a support for nanosized Mn oxide was used toward the synthesis of a new catalyst for water oxidation. A composite of Mn and Ho oxide was synthesized by a simple hydrothermal procedure and characterized by scanning electron microscopy, extended edge X-ray absorption fine structure, transmission electron microscopy, powder X-ray diffraction and electrochemical methods. The effect of calcination temperature on the composite performance for the water-oxidation process was examined in the range of 100–600 °C. The turnover frequencies for the catalyst toward water oxidation in the presence of cerium(IV) ammonium nitrate and photo-produced tris(2,2′-bipyridyl)ruthenium(III), [Ru(bpy)3]3+ are 0.12 and 0.04 (mmol O2 per mol Mn s), respectively. In addition, the catalyst shows self-healing in the presence of cerium(IV) ammonium nitrate and under the water-oxidation conditions.


 Please wait while we load your content...
Please wait while we load your content...