Synthesis of 5,5′-azoxybistetrazole via nitration and de-oxygen rearrangement of triazene†
Abstract
An efficient approach to synthesize 5,5′-azoxybistetrazoles has been achieved via the treatment of tetrazolyl triazenes with fuming nitric acid and acetic anhydride in a two-step one-pot reaction. Herein, 5,5′-azoxybistetrazole is proposed to be formed via a nitroso triazene intermediate generated from tetrazolyl triazene by nitration and de-oxygen rearrangement. All compounds were fully characterized using IR, 1H and 13C NMR spectroscopy, and HRMS. In the case of 2,2′-dimethyl-5,5′-azoxybistetrazole (2a), single crystal X-ray structuring and 15N NMR spectroscopy were also performed. The calculations predict that 2f has a detonation velocity of 8066 m s−1 and a detonation pressure of 25.8 GPa.
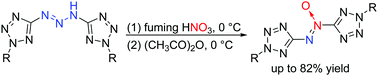


 Please wait while we load your content...
Please wait while we load your content...