A benzopyrylium–phenothiazine conjugate of a flavylium derivative as a fluorescent chemosensor for cyanide in aqueous media and its bioimaging†
Abstract
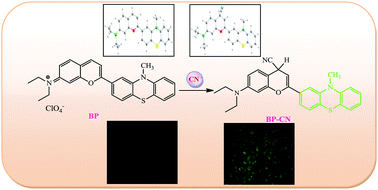
A new benzopyrylium–phenothiazine conjugate (BP) of a flavylium derivative was synthesized and characterized. The probe BP was shown to be selective and sensitive for CN− among the 17 anions and biothiols studied in HEPES buffer medium by fluorescence, absorption, and visual color change. The colorimetric and fluorescence response of the probe BP to cyanide ions is due to the Michael addition of cyanide to the activated Michael receptor of the probe which blocks an intramolecular charge transfer process. The probe displays a fast response to cyanide ions at room temperature, and a maximal fluorescence signal is achieved in the presence of only 2 equivalents of cyanide ions. Moreover, the probe BP could be used as a practical, visible colorimetric test strip for CN− in an aqueous environment. TDDFT calculations were performed in order to demonstrate the electronic properties of the probe and its cyanide product. Density functional reactivity theory (DFRT) calculation also exhibits the characterisation of the most electrophilic and nucleophilic centres of the molecule. The probe could be applied for imaging cyanide in live cells by fluorescence imaging.


 Please wait while we load your content...
Please wait while we load your content...