Impact of linker between triazolyluracil and phenanthridine on recognition of DNA and RNA. Recognition of uracil-containing RNA†
Abstract
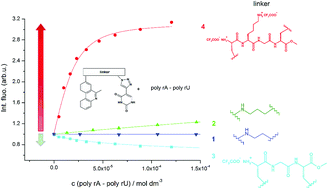
A new group of multifunctional ligands for DNA and RNA were prepared, comprising phenanthridinium and triazolyluracil connected by various aliphatic or peptide linkers. The peptide linker conjugates showed at least one order of magnitude higher affinity in comparison to aliphatic analogues, which was attributed to peptide-specific hydrophobic and hydrogen bonding interactions within DNA/RNA binding sites. Particularly, a peptide linker conjugate with lysine residue showed selectivity toward poly rA–poly rU, as well as toward poly U, both characterized by strong affinity and a selective fluorescence response. Negligible cytotoxicity of compounds additionally supported their possible applications as fluorimetric probes in laboratory use.


 Please wait while we load your content...
Please wait while we load your content...