Selection of catalytically active elements for removing NO and CO from flue gas at low temperatures
Abstract
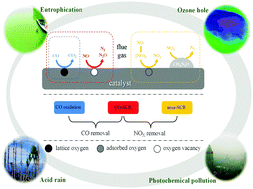
Selective catalytic reduction of nitrogen oxides with carbonic oxide (CO-SCR) has been suggested as an attractive and promising method for removing NO and CO simultaneously from flue gas. Nevertheless, the presence of oxygen has an inhibitory effect on the catalytic reaction. Based on CO-SCR on a urea modified metal oxide catalyst, a novel method was proposed to achieve NO and CO removal under oxygen-rich conditions via heterogeneous catalysis (CO-SCR, CO oxidation and urea-SCR). The selection of suitable active metal elements for catalysts is the pivotal issue in present research. This paper shows a comprehensive thermodynamic study for screening active metals through studying the properties of 28 different metal oxide reduction reactions and 11 different oxidation reactions at different temperatures referring to 20 preselected elements. This research mainly includes thermodynamic equilibrium calculations at different oxygen concentrations, detailed analyses of the NO and CO removal mechanism and thermodynamic screening of active metal elements based on the Gibbs free energy change (ΔG) at different temperatures. On the basis of the systematic thermodynamic study, the metal elements Mn, Cu, Pb and V have been selected as suitable for removing NO and CO from flue gas at low temperatures (50–120 °C).


 Please wait while we load your content...
Please wait while we load your content...