Visual and optical detection of hypochlorite in water samples based on etching of gold/silver alloy nanoparticles†
Abstract
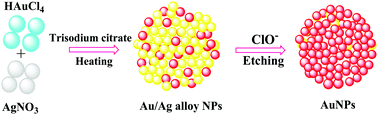
In this work, we have described a cost-effective, simple, selective and sensitive approach for the detection of hypochlorite (ClO−) using gold/silver alloy nanoparticles (Au/Ag alloy NPs) as a colorimetric probe. The synthesized Au/Ag alloy NPs were characterized by UV-Vis absorption, zeta potential and high-resolution transmission electron microscopy (HR-TEM) measurements. Formation of homogeneous alloy NPs was evidenced from a single surface plasmon resonance (SPR) band at 410 nm in absorption spectral measurements. Upon the successive addition of ClO−, the absorption intensity of Au/Ag alloy NPs showed a drastic decrease accompanied by a significant red shift from 410 nm to 522 nm along with a notable change in color from yellow to purple. These observed color and spectral changes are due to the strong oxidizing nature and etching effect of ClO− on the surface of the Au/Ag alloy NPs. The present sensing method was able to identify ClO− from other interfering anions and cations with a limit of detection of 0.30 × 10−6 mol dm−3 (S/N = 3) in the linear range from 2.40 to 24.00 × 10−6 mol dm−3. The assay was successfully utilized for sensing of ClO− in tap water samples.


 Please wait while we load your content...
Please wait while we load your content...