Synthesis and characterization of chiral and achiral diamines containing one or two BODIPY molecules†
Abstract
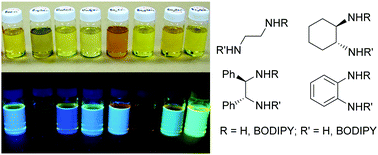
The synthesis and characterization of one or two BODIPY fragments appended to four new chiral and one new achiral diamines is described. All of the examined BODIPY-appended diamines exhibit a quasireversible-irreversible reduction, with two reductions (separated by about 100 mV) observed in the case of diamines containing two BODIPY molecules. Only the BODIPY-appended ortho-phenylenediamines did not fluoresce under UV light. Computational analysis showed that the absence of fluorescence of the BODIPY-appended ortho-phenylenediamines is likely due to intramolecular quenching of the excited state electron within the phenylenediamine ligand. Computational analysis also showed that the incorporation of a BODIPY molecule greatly reduces the basicity of the amine center, by about 10–14 pKa units. The BODIPY moiety was found to be more electron withdrawing than a tosyl and a pentafluorophenyl group, suggesting why excess metals are needed in heavy metal sensor applications (heteroatom-appended BODIPYs = poor ligands). An improved procedure for the scalable synthesis (greater than three grams) of 8-methanethio-BODIPY, a common starting material for the generation of heteroatom-appended BODIPY molecules, is also described.


 Please wait while we load your content...
Please wait while we load your content...