Carbazole- and/or triphenylamine-based D–π–D multiarylamino dyes: synthesis, characterization and photophysical properties†
Abstract
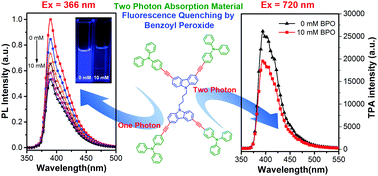
We report herein the design and synthesis of a class of dipolar and quadrupolar carbazole- and/or triphenylamine-based D–π–D multiarylamino dyes (DCzBus), which contain a 1,4-butylidene biscarbazole core linked to hexylcarbazole or triphenylamine peripheral groups through alkynyl bridges. For multiarylamino groups and bulk conjugated structure, DCzBus all show strong one-photon and two-photon excited blue-violet fluorescence emission bands from 350 to 480 nm. Especially, DCzBus with triphenylamine peripheral groups have higher fluorescence quantum efficiency and longer fluorescence lifetime than those with carbazole peripheral groups. Stern–Volmer measurement indicated that these DCzBus could show fluorescence quenching response to benzyl peroxide through the photo-induced electron transfer mechanism. The fluorescence quantum yield and lifetime and non-planar conformation of DCzBus could significantly affect their photo-induced electron transfer process with benzoyl peroxide. Two-photon excited fluorescence quenching under different excited laser wavelengths was observed, which indicated that the D–π–D multiarylamino dyes designed in this study are potentially useful two-photon-induced fluorescence probes.


 Please wait while we load your content...
Please wait while we load your content...