The nature of the Au–N bond in gold(iii) complexes with aromatic nitrogen-containing heterocycles: the influence of Au(iii) ions on the ligand aromaticity†
Abstract
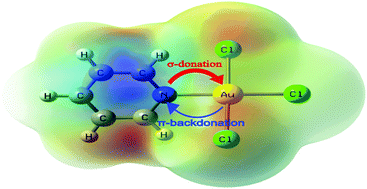
In this work, the nature of the bonding interaction between Au(III) ion and aromatic nitrogen-containing heterocycles (N-heterocycles) in a series of Au(III) complexes was studied by means of the natural bond orbital (NBO), atoms in molecule (AIM), charge decomposition analysis (CDA) and energy decomposition analysis (EDA) methods at the B3LYP/cc-pVTZ + LanL2TZ(f) level of theory. It was found that the Au–N coordinative bond has a higher electrostatic than covalent character. The studied N-heterocycles were found to be strong σ-electron donors, but rather weak π-electron acceptors. In addition, the local aromatic distribution in the studied N-heterocycles, and in their Au(III) complexes, was quantified through several different aromaticity indices: the six center delocalization index (SCI), the harmonic oscillator model of aromaticity (HOMA) index and the nucleus-independent chemical shifts (NICS). It was shown that the aromatic character of the nitrogen-containing ring in gold(III) complexes is decreased in comparison with the same ring of uncoordinated ligands.


 Please wait while we load your content...
Please wait while we load your content...