A simple one-pot synthesis of a Zn(O,S)/Ga2O3 nanocomposite photocatalyst for hydrogen production and 4-nitrophenol reduction†
Abstract
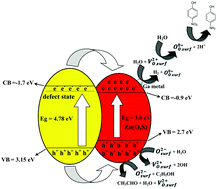
Noble metal-free Zn(O,S)/Ga2O3 nanocomposite photocatalysts containing different amounts of Ga2O3 have been synthesized using a precipitation method at 90 °C. The as-prepared catalysts were characterized and their composite nature was confirmed. The Zn(O,S)/Ga2O3 catalysts were examined for their ability to evolve hydrogen under low UV light illumination (0.088 mW cm−2) and to provide hydrogen for 4-nitrophenol (4-NP) reduction. Gas chromatography measurements revealed that hydrogen was produced at a rate of 280 μmol g−1 h−1 W−1, while UV-vis absorption and high-performance liquid chromatography (HPLC) measurements confirmed the formation of 4-aminophenol (4-AP) as a product of 4-NP reduction. The heterojunction formation between the Zn(O,S) and Ga2O3 phases enhanced the photocatalytic activity, compared to the single Zn(O,S) and Ga2O3 phases. Surface oxygen anions and oxygen vacancies played important roles in the photocatalytic mechanism to evolve hydrogen and to utilize hydrogen ions in 4-NP reduction. The photocatalytic hydrogen evolution reaction and its utilization for 4-NP reduction to 4-AP were evaluated and elucidated in this work.


 Please wait while we load your content...
Please wait while we load your content...