Substituent effects on the basicity (pKa) of aryl guanidines and 2-(arylimino)imidazolidines: correlations of pH-metric and UV-metric values with predictions from gas-phase ab initio bond lengths†
Abstract
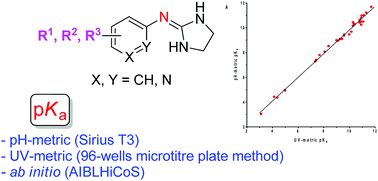
The dissociation constants of two related series of 2-(arylimino)imidazolidine and aryl guanidine α2-adrenoceptor antagonists (35 compounds in total) were measured by potentiometric titrations and by UV-spectrophotometry using the 96-well microtitre plate method. The experimental values obtained using both methods were quite consistent and showed a very good agreement with the pKa values calculated using the AIBLHiCoS methodology, which uses only a single bond length obtained using ab initio calculations at a low level of theory. The prediction power of the imidazolidine and guanidine set of compounds was very good with deviations typically <0.30 and <0.24 pKa units, and a mean absolute error (MAE) of 0.23 and 0.29, respectively. The study of the quantitative effect of diverse substituents on the basicity of aryl guanidine and 2-(arylimino)imidazolidine derivatives is useful for medicinal chemists working with biologically relevant guanidine-containing molecules.


 Please wait while we load your content...
Please wait while we load your content...