Nickel-catalyzed reductive defunctionalization of esters and amides to aromatic hydrocarbons
Abstract
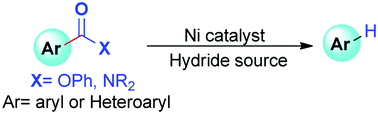
The removal of ester and amide groups is of fundamental significance in organic syntheses. Under non-catalytic conditions, hydride sources are chiefly used for their reduction. Recently developed Ni-catalyzed one-pot reductive activation of esters and amides followed by tandem C–CO bond cleavage–decarbonylation facilitates their cleavage to aromatic hydrocarbons. Isolation and characterization of key reaction intermediates provide insight into this acyl C–O bond activation pathway.
- This article is part of the themed collection: 2017 Focus and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...