Csp2–Br bond activation of Br-pyridine by neophylpalladacycle: formation of binuclear seven-membered palladacycle and bipyridine species†
Abstract
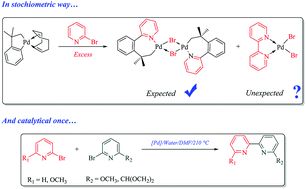
In this work, the synthesis and reactivity of seven-membered palladacycles are described, and a novel bi-pyridine synthesis in a catalytic pathway is reported. Neophyl-palladacycle(I) reacts with an excess of 2-Br-pyridine, giving the desired new binuclear seven-membered palladacycle (1) and unexpectedly, a bipyridine complex, [Pd(BiPy)Br2]. ESI-HRMS experiments show that fragmentation of the Pd–Br bond in 1 can take place producing unusual two coordinated Pd(II) molecular ions, [Pd(NeoPyR)]+.


 Please wait while we load your content...
Please wait while we load your content...