New insights into the influence of weak and strong acids on the oxidative stability and photocatalytic activity of porphyrins†
Abstract
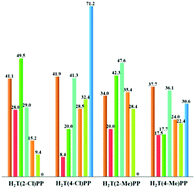
A series of electron-rich and electron-demanding meso-tetra(aryl)porphyrins (aryl = phenyl, 4-methoxyphenyl, 4-methylphenyl, 4-chlorophenyl, 2-methylphenyl and 2-chlorophenyl) and their dications with weak and strong acids (CF3COOH, CHCl2COOH, CH2ClCOOH, HCOOH, HCl and HClO4) were used as photosensitizers for the aerobic photooxidation of cyclooctene under homogeneous conditions. Mechanistic studies in the presence of a singlet oxygen quencher (1,3-diphenylisobenzofuran) and 1,4-benzoquinone as the superoxide anion radical scavenger confirmed the involvement of a singlet-oxygen mechanism in the oxidation reactions. Also, the solvent effects on the catalytic activity of the porphyrin diacids were explained by the relative lifetimes of singlet oxygen in different solvents. The formation of dications was found to either increase or decrease the photocatalytic activity, photostability and singlet oxygen quantum yield (ϕΔ) of porphyrins. The non-radiative rate constants (knr) for the decay of the S1 excited state of the photosensitizers were often greater in the case of porphyrin diacids. The ϕΔ values of the porphyrins and dications were in the range of 0.48–0.89 and 0.06–0.98, respectively. Interestingly, despite the large ϕΔ value (0.98) found for H4T(4-OMe)PP(CF3COO)2 and H4T(4-OMe)PP(HCOO)2, H4T(4-Cl)PP(HCOO)2 (ϕΔ = 0.29) showed the highest catalytic activity of the series. This observation was attributed to extensive oxidative degradation of the former. It is noteworthy that the ϕΔ parameters were evaluated in a short reaction time (ca. 20 min) and therefore no detectable catalyst degradation was observed in this time interval. Furthermore, H4TPP(HCOO)2 was the most stable photocatalyst under the reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...