Copper-catalyzed aerobic oxidative intramolecular amidation of 2-aminophenylacetylenes: a domino process for the synthesis of isatin†‡
Abstract
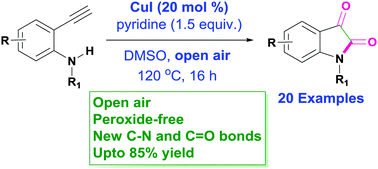
A novel CuI-catalyzed oxidative amidation of 2-aminophenylacetylenes leading to the formation of isatins by using open air as an oxygen source has been developed. The reaction proceeded smoothly and provided a variety of isatin derivatives in good yields. The advantages of this one-pot reaction protocol include the use of a green oxidant, low-price catalyst and facile conditions, and easy handling.


 Please wait while we load your content...
Please wait while we load your content...