Catalytic reduction of NO by CO molecules over Ni-doped graphene: a DFT investigation†
Abstract
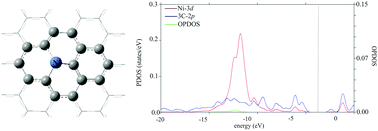
The aim of the present study is to study the catalytic activity of Ni-doped graphene in the reduction of NO by CO molecules. The adsorption energies, geometric parameters, reaction barriers, and thermodynamic properties are calculated using the M06-2X density functional. The results suggest that the reaction proceeds in two steps, initiated by the co-adsorption of two CO molecules over the surface, followed by the addition of NO molecules to form a stable (OCON)2 intermediate. Next, by overcoming a small activation energy, a N2 molecule is formed which can easily desorb from the surface due to its negligible adsorption energy. Finally, two CO2 molecules are formed over the surface, which need a negligible activation energy. According to our results, Ni-doped graphene can be used as a potential catalyst for NO reduction by CO molecules. These theoretical results could be also useful in practical applications for the removal of toxic NO and CO molecules.


 Please wait while we load your content...
Please wait while we load your content...