Borylation, silylation and selenation of C–H bonds in metal sandwich compounds by applying a directing group strategy†
Abstract
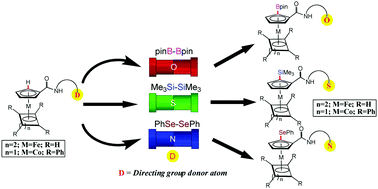
Palladium-mediated borylation, silylation, and selenation have been achieved by C–H activation using bidentate directing groups linked on iron and cobalt sandwich compounds. Herein, the first examples of borylation and silylation using pyridine-N-oxide and (2-methylthio)phenyl directing groups have been shown using Pd(OAc)2 as a catalyst. In the case of C–H selenation, the product was formed by treating diphenyl-diselenide with an 8-aminoquinoline-based palladacycle. The novel palladacycle intermediates of the C–B, C–Si, and C–Se bond formation have been isolated and structurally characterized. A correlation between the type of heteroatom added and the donor atom of the installed bi-dentate directing group has also been presented.


 Please wait while we load your content...
Please wait while we load your content...