Perylene diimide-based organic π-motif for differentiating CN− and F− ions by electron-transfer and desilylation mechanisms: applications to complex logic circuits†
Abstract
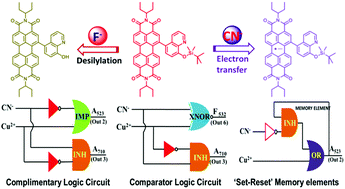
In this work, we have designed and synthesized a perylene diimide based derivative PDI-SiHQ having an O-silylated-8-hydroxyquinoline group at the bay position. PDI-SiHQ is able to detect and differentiate CN− from F− in the presence of other anions. The electron transfer from cyanide ions to PDI-SiHQ leads to the emergence of panchromatic and NIR absorption bands due to the formation of a PDI-SiHQ˙− radical anion. In contrast, fluoride induced desilylation of PDI-SiHQ coupled with π–π aggregation results in a red-shifted absorption band at 730 nm. 1H NMR and cyclic voltammetry combined with the reversal of the PDI-SiHQ˙− radical anion to the PDI-SiHQ neutral form in the presence of NOBF4 or Cu2+ ions confirm the formation of the radical anion in PDI-SiHQ. Multi-channel UV-Vis-NIR absorption and emission bands and their reversibility with NOBF4/Cu2+ ions offer the possibility for fabrication of complex logic circuits such as complementary IMP/INH logic circuits, magnitude comparators and ‘set–reset’ flip flop memory elements.


 Please wait while we load your content...
Please wait while we load your content...