Silver-induced electronic drift in AgPd bimetallics: rationale for enhanced electrocatalytic activity of ethanol oxidation reaction†
Abstract
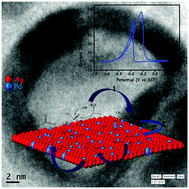
Motivated by the possible synergistic electronic effect observed in the Ag and Pd system, simple galvanic replacement reaction (GRR) is carried out for the large scale fabrication of bimetallic AgPd nanoparticles using a modified hydrothermal (MHT) technique. The typical galvanic replacement process under MHT drives the evolution process for AgCl free etched AgPd nanostructure. In the reaction, a sacrificial Ag nanoparticle template in a surfactantless condition is consumed quantitatively depending on the Pd2+ ion concentration. Verification of the synthetic technique has been proved by the change in the nature of the surface plasmon resonance (SPR) peak of the Ag nanoparticle. The as-synthesized unsupported AgPd nanostructure exhibits a superior electrochemical property towards the ethanol oxidation reaction (EOR) in an alkaline medium in comparison to electrochemically active commercial Pd/C. The enhancement of the catalytic Pd activation relates to its active sites in the presence of Ag. The presence of oxophilic Ag and synergism between Ag and Pd drastically reduces the catalyst poisoning effect, explained by the electron affinity and electronegativity parameters of the participating metals. The Ag-driven electron drift from Pd and the Ag/Pd composition variation successfully illustrate the EOR mechanism.


 Please wait while we load your content...
Please wait while we load your content...