Synthesis, G-quadruplex binding properties and cytotoxicity of naphthalimide–thiourea conjugates†
Abstract
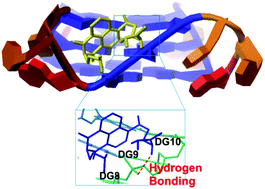
Four novel naphthalimide–thiourea conjugates 3a–d have been prepared and characterized. The interactions of these compounds with duplex and telomeric G-quadruplex DNA have been investigated by UV-Vis spectroscopy, fluorescence spectroscopy, viscosity and cyclic voltammetric measurements. The studies reveal that 3a–d possess high affinity (>1.20 × 107 M−1) and reasonable selectivity for telomeric G-quadruplex DNA over duplex DNA. Viscosity and cyclic voltammetric experiments suggest that 3a–d bind to duplex DNA through a classical intercalation mode. Molecular docking studies indicate that 3a–d associate with telomeric G-quadruplexes through groove binding, and hydrogen bonding interactions exist between the thiourea moiety and the phosphates or the bases in the G-quadruplex. The emissive nature of compounds 3a–d makes it possible to study their localization in A549 cells by fluorescence microscopy. The representative compounds 3d and 3b are mainly localized in the nuclei after 4 h of incubation. All four compounds inhibit A549 cells selectively over normal HUVEC cells, with a higher antitumor activity than amonafide.


 Please wait while we load your content...
Please wait while we load your content...