Synthesis, crystal structures, molecular docking and in vitro cytotoxicity studies of two new copper(ii) complexes: special emphasis on their binding to HSA†
Abstract
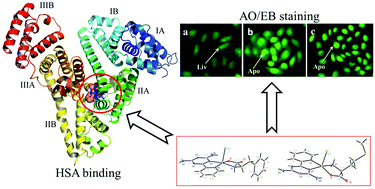
Two new mixed-ligand copper(II) complexes, [Cu(PyTA)(L-Phe)Cl]·2H2O (1), [Cu(PyTA)(L-Met)Cl]·0.5H2O (2) (where PyTA = 2,4-diamino-6-(2′-pyridyl)-1,3,5-triazine, L-Phe = L-phenylalanine, L-Met = L-methionine), were synthesized and characterized by elemental analysis, IR, UV-vis, molar conductivity, ESI-MS, and X-ray crystal diffraction. The copper centers of complexes 1 and 2 are in slightly distorted square pyramidal environments, and fluorescence quenching experiments and calorimetry showed that the complexes bound to human serum albumin (HSA) with high affinities (binding constants KD = 3.71 × 106 L mol−1 for 1 and 1.99 × 106 L mol−1 for 2, 1 > 2). UV-vis and circular dichroism (CD) spectroscopic investigations revealed that the complexes induced the alteration of hydrophobic environments and the decrease in the α-helix level of HSA during binding processes. Synchronous fluorescence spectra demonstrated that the binding sites of the complexes on HSA were close to the Trp-214 residue in the IIA subdomain, which was further verified by site marker competitive experiments and the molecular docking method. In vitro cytotoxicity experiments showed that the effective HSA-binding interactions enhanced the cytotoxicity of the complexes. Both 1 and 2 displayed strong anticancer activities against the Eca-109 cell line and induced cell death through the apoptosis pathway.


 Please wait while we load your content...
Please wait while we load your content...