Competing phenol–imidazole and phenol–phenol interactions in the flexible supramolecular environment of N,N′-bis(3-imidazol-1-ylpropyl)naphthalenediimide causing domain expansion†
Abstract
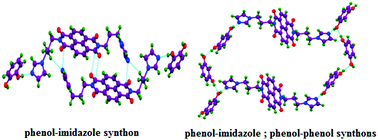
The relevance of a fundamentally important imidazole⋯phenol interaction in the self-assembly of cocrystals and a salt of a bis-imidazole hinged through a flexible tether to a naphthalenediimide, namely N,N′-bis(3-imidazol-1-ylpropyl)naphthalenediimide (L), with a series of phenolic compounds is studied. Cocrystals of L with phenolic compounds, 1,2-dihydroxybenzene (cat), 1,3-dihydroxybenzene (res), and 1,3,5-trihydroxybenzene (phi), namely L·cat, L·(res)2, L·phi·H2O, and a salt with 2,4-dinitrophenol (dnp), L2+·(dnp−)2, are structurally characterized. Hierarchical effects of oxime-imidazole synthons in the cocrystal of L with 2,3-dihydroxyphenylaldoxime, L·(dhpa)2, are also investigated. The cocrystals of L with cat and phi are formed in a 1 : 1 molar ratio, but the latter is a hydrated cocrystal. Compound L adopts a C-like shape in cocrystals L·cat and L·phi·H2O, whereas L adopts an S-like shape in the L·(res)2 cocrystal. It adopts an I-like shape in the salt L2+·(dnp−)2 or in the cocrystal L·(dhpa)2. Analyses of cocrystals of L with these phenolic compounds show a significant presence of charge-transfer interactions between naphthalenediimide only with phi. Phenol⋯imidazole interactions together with C–H⋯N(imidazole) and N⋯π interactions cause domain expansion in L·cat by forming dimeric sub-assemblies providing C-like geometry to L. In this example, one of the flexible arms bearing imidazole is crystallographically disordered, and a low temperature 1H-NMR study reveals conformational changes. In contrast, in the case of L·phi·H2O, domain expansion that takes place due to the interplay of charge-transfer, imidazole⋯phenol and phenol⋯water interactions provides a C-like shape to L. In these cases, phenol⋯phenol interactions have less relevance. In the case of the 1 : 2 salt L2+·(dnp−)2, imidazolium⋯phenolate interactions dominate and provide a stretched geometry to L, whereas competition to form homodimers between oximes and oxime⋯imidazole interactions predominates in the cocrystal L·(dhpa)2. This cocrystal has a stretched structure of L. In the lattice of this cocrystal, phenol⋯phenol interactions are not relevant. Cocrystal L·res is identified as one exception among the series where competition between phenol⋯phenol with phenol⋯imidazole interactions results in domain expansion facilitating an interpenetrated structure.


 Please wait while we load your content...
Please wait while we load your content...