Improving photocatalytic reduction of 4-nitrophenol over ZrO2–TiO2 by synergistic interaction between methanol and sulfite ions†
Abstract
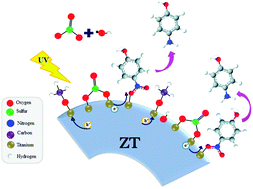
The effect of two sacrificial agents (methanol and sodium sulfite) on the photocatalytic reduction of 4-nitrophenol employing a ZrO2–TiO2 photocatalyst is reported. The experimental results showed a decrease in the generation of OH˙ radicals and diminution in the charge transfer resistance that favor the electron transfer toward 4-nitrophenolate ions. Based on the results, it is possible to establish that methanol acts as a hole scavenger while sulfite ions act as radical scavengers, improving the reduction from 4-nitrophenol to 4-aminophenol. The synergistic effect has been corroborated by photoluminescence and photoelectrochemical measurements.


 Please wait while we load your content...
Please wait while we load your content...