Influence of Ca doping and calcination temperature on selective catalytic oxidation of NO over Mn–Ca–Ox–(CO3)y catalysts†
Abstract
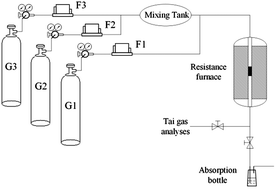
Selective catalytic oxidation (SCO) is an unconventional technology for denitration. The key to the SCO approach is to develop a novel catalyst that can achieve high catalytic oxidation activity at low temperature. A series of Mn–Ca–Ox–(CO3)y catalysts were prepared by co-precipitation methods and used for the catalytic oxidation of nitric oxide (NO) over a low temperature range (i.e., 60–360 °C). The results indicated that the best NO conversion over the Mn–Ca–Ox–(CO3)y catalysts was 79.4% at 270 °C with a space velocity of 30 000 h−1. By comparing the activities of MnOx and Mn–Ca–Ox–(CO3)y, the activities of MnOx are lower than those of Mn–Ca–Ox–(CO3)y, especially in the low temperature region (<240 °C). Therefore, doping with calcium could enhance the catalytic oxidation activity. The addition of calcium plays an active role in MnO2 generation and the structural properties of the catalysts. The characterization results indicated that the MnO2 that was generated at a low calcination temperature was favourable for oxidation of NO. The in situ diffuse reflectance infrared transform spectroscopy (DRIFTS) results indicated that nitrate could be more easily generated on Mn–Ca–Ox–(CO3)y due to alkaline oxide existing in the catalysts, which is the primary reason why the Mn–Ca–Ox–(CO3)y catalysts exhibit higher activity compared to MnOx, especially at low temperature.


 Please wait while we load your content...
Please wait while we load your content...