Nanorod TiO2 sensor for dopamine: a voltammetric study
Abstract
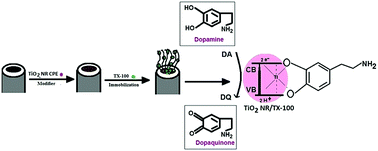
Nanorod shaped titanium oxide (TiO2NRs) was synthesized by the hydrothermal process in 10 M NaOH(aq.) at 120 °C. The TiO2NRs were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), infrared spectroscopy (IR) and UV-visible absorption spectroscopy (UV-vis). An electrochemical sensor for the responsive determination of dopamine (DA) was evolved by designing a carbon paste electrode (CPE) modified with titanium oxide nanorods (TiO2NRs/MCPE). The modification of the TiO2NRs using different surfactants like sodium dodecyl sulphate (SDS), cetyltrimethylammonium bromide (CTAB) and polyglycine failed to produce an impressive charge transfer and the resulting electrodes were also less sensitive for the determination of DA. However, modification with iso-octyl phenoxy polyethoxy ethanol (TX-100) improved the redox property of DA, with increased current intensity on the surface of the TiO2NRs/MCPE, showed good electrocatalytic properties and also produced an enhancement in the peak currents for DA (TiO2NRs/TX-100/MCPE). The detection limit of DA was determined from a differential pulse voltammetric (DPV) study and was found to be 42 × 10−9 M. The effect of concentration, scan rate and pH of DA was studied and a simultaneous determination of dopamine (DA), uric acid (UA), ascorbic acid (AA) and paracetamol (PA) using the TiO2NRs/TX-100/MCPE was carried out. The interference studies showed that the modified electrode exhibits excellent selectivity in the presence of a large excess of interferents and the response is fast, stable and can be applied for real sample analysis in medical, biotechnological and pharmaceutical fields.


 Please wait while we load your content...
Please wait while we load your content...