Structural and thermodynamic investigation of AnIVLI(O)HOPO
Abstract
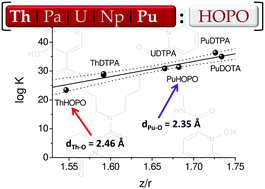
For the first time, capillary electrophoresis coupled with inductively coupled plasma mass spectrometry has been used to determine the stability constants of PuIV with the multidentate hydroxypyridinonate chelating agents 3,4,3-LI(1,2-HOPO) and 5-LIO(Me-3,2-HOPO) in 0.1 M NaNO3 solution, pcH = 1.395 at 25 °C through competition with the NTA ligand. The limiting electrophoretic mobility was found to be zero for AnIV[5-LIO(Me-3,2-HOPO)] and slightly positive for AnIV[3,4,3-LI(1,2-HOPO)] (AnIV = Th, Pu). They were respectively assigned to the formation of the 1 : 2 neutral species An[5-LIO(Me-3,2-HOPO)]2 and a mixture of the neutral species AnIV[3,4,3-LI(1,2-HOPO)] and its protonated form AnIVH[3,4,3-LI(1,2-HOPO)]+. The corresponding stability constants of ThIV with both chelators were evaluated through the same experiments for the sake of comparison. The stability of both PuIV–HOPOs was about ten orders of magnitude better than that of the equivalent ThIV complexes. To complement these thermodynamic data, structural parameters of Pu[3,4,3-LI(1,2-HOPO)] and Th[3,4,3-LI(1,2-HOPO)] complexes in solution have been derived from EXAFS experiments and compared to previously reported crystallographic structures.


 Please wait while we load your content...
Please wait while we load your content...