Preparation of highly mesoporous honeycomb-like TiO2 and its excellent application†
Abstract
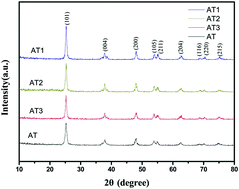
TiO2 has poor photocatalytic performance when synthesised using traditional processes under visible light, which has limited its practical applications. In this work, titanium sulfate and hydrazine hydrate were used as raw materials to fabricate highly mesoporous honeycomb TiO2via a two-step hydrothermal and calcination method. The formation mechanism of the highly mesoporous TiO2 was analyzed based on experiments and theories. The parameters were flexible using the preparation method. Samples with high photocatalytic performance can be obtained without strictly controlling the process parameters such as hydrazine hydrate dosage, the hydrothermal temperature and so on. The preparation process was as follows: the raw materials were prepared at 180 °C for 48 h in the first hydrothermal stage, and then synthesized at 150 °C for 48 h in the second hydrothermal process. The hydrazine hydrate dosage was 10 ml in the second process. The prepared grain sizes were about 16.4 nm and the specific surface areas were approximately 105.12 m2 g−1 after the samples were heated at 450 °C for 4 h. Their degradation rate was 1.8 times that of P25. The improved performances are ascribed to the unique point defects because of nitrogen atom doping in the TiO2 and the highly mesoporous structure increased by adding hydrazine hydrate.

 Please wait while we load your content...
Please wait while we load your content...