Thermodynamic modeling and experimental verification of a NaNO3–KNO3–LiNO3–Ca(NO3)2 system for solar thermal energy storage
Abstract
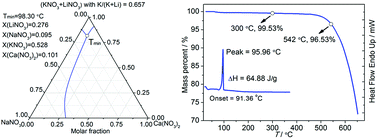
A quaternary system, NaNO3–KNO3–LiNO3–Ca(NO3)2, was predicted using the extended symmetric Kohler model to be a molten mixture with a low melting point that can be used as a heat storage and transfer fluid in concentrating solar power. The predicted eutectic temperature and composition of this system are 98.30 °C and 9.5 mol% NaNO3, 52.8 mol% KNO3, 27.6 mol% LiNO3 and 10.1 mol% Ca(NO3)2. Differential scanning calorimetry and thermogravimetric analysis were used to measure the predicted eutectic mixture. The results showed that the salt mixture has a low melting point of 95.96 °C, which coincides with the predicted eutectic temperature (98.30 °C), a moderate heat of fusion of 64.88 J g−1, and a thermal stability temperature of 450 °C. Therefore, this quaternary eutectic salt is suitable for use as a heat storage and transfer medium in solar thermal power plants.


 Please wait while we load your content...
Please wait while we load your content...