Molecular modelling approach to elucidate the thermal decomposition routes of vanillin†
Abstract
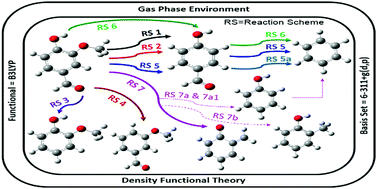
The presence of very high amounts of oxy-components in unprocessed bio-oil after thermochemical conversion of lignocellulosic biomass is an undesirable property of bio-oil. This results in severe drawbacks for raw bio-oil which are considered to be undesirable characteristics of any fuel, e.g., low heating value, corrosiveness, high viscosity, and low stability. Therefore, it is necessary to eliminate oxygen atoms from the components of bio-oil. On the other hand, the very high number of oxy-components in unprocessed bio-oil offers an excellent platform to acquire various specialty chemicals. Therefore, in this work, vanillin (4-hydroxy-3-methoxy-benzaldehyde) is considered as a model compound of lignin-derived bio-oil, and various chemical conversions are conducted to achieve lower molecular weight hydrocarbon fractions and several important intermediates, e.g., benzene, guaiacol, o-cresol, p-hydroxybenzaldehyde, m-methoxybenzaldehyde, phenol and o-quinonemethide. Bond dissociation energy studies were carried out to observe the potential chemical breakage sites of vanillin. Chemical reaction mechanisms are proposed according to the various bond dissociation possibilities, and potential energy surfaces are reported for each reaction scheme. The production of guaiacol from vanillin using atomic hydrogenation at the aromatic carbon of the Caromatic–CHO bond of vanillin followed by formyl group removal was found to be the pathway requiring the lowest activation energy (only 10.13 kcal mol−1). The present results are in accordance with their experimental counterparts wherever applicable. The thermochemical phenomena of these reactions were studied in a wide temperature range, i.e., 598 to 898 K for the gas phase and 298 to 498 K for the aqueous phase, at a fixed pressure of 1 atm. The aqueous phase environment was created by a SMD model using water as the solvent. All reaction schemes in both phases were favourable under all temperature conditions except for the formation of phenol from vanillin via the formation of 5-formylsalicylaldehyde, as reported in reaction schemes 7a and 7a1.


 Please wait while we load your content...
Please wait while we load your content...