An efficient catalyst-free one-pot synthesis of primary amides from the aldehydes of the Baylis–Hillman reaction†
Abstract
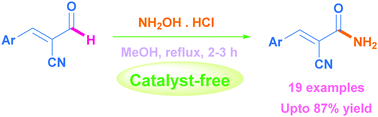
Herein, a facile and efficient method for the preparation of allyl amides from the aldehyde of Baylis–Hillman adducts has been developed using a hydroxylamine/methanol system under a catalyst-free condition. The effects of solvents and temperature on the reaction and substituents on the phenyl ring have been examined. This method is best demonstrated by its advantages such as operational simplicity, moderate to excellent yields, short reaction time, and simple reaction procedure. Most importantly, the reaction proceeds smoothly in the absence of a catalyst and an external oxidant.


 Please wait while we load your content...
Please wait while we load your content...