Behavior, mechanism, and equilibrium studies of rhodium(i) extraction from hydrochloric acid with HMImT
Abstract
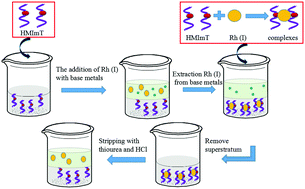
1-Hexyl-3-methylimidazole-2-thione (HMImT), a low-viscosity extractant, was firstly synthesized and used to extract rhodium(I). The negligible solubility of HMImT in solution was measured by HPLC to be about 0.015%. In the extraction process, rhodium(III) in solution was activated into rhodium(I) by SnCl2 to improve extraction percentage, which was confirmed by XPS spectra. Under optimum conditions, the extraction percentage reached 99.6%. The neutral complexing mechanism was confirmed by UV-vis spectra, 1H NMR spectra, titration method and FT-IR spectra. Quantum chemical calculations based on density functional theory (DFT) were also performed to support the results from a theoretical perspective. The extraction process was modeled by Langmuir (R2 = 0.998), Freundlich (R2 = 0.972) and Dubinin–Radushkevich isotherms (R2 = 0.990). According to the model results, the monolayer absorption capacity was 2.82 mmol g−1, and the mean free energy was obtained as 9.8 kJ mol−1. The thermodynamic parameters of ΔG, ΔH and ΔS showed that the extraction process was spontaneous and exothermic. Pseudo-second-order kinetics well fitted the experimental data (R2 = 0.996). In addition, HMImT possesses high selectivity to Rh(I) rather than other base metals during extraction. In summary, HMImT with low dissipation, excellent extractability and high selectivity for rhodium(I) extraction will hold great promise and potential in the separation field.


 Please wait while we load your content...
Please wait while we load your content...