One-pot synthesis of N-substituted pyrroles from nitro compounds and 2,5-hexadione over a heterogeneous cobalt catalyst
Abstract
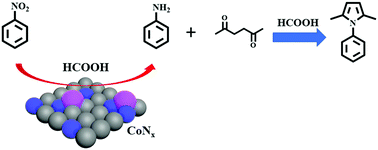
In this study, the one-pot heterocyclization of nitro compounds with 2,5-hexadione was studied for the synthesis of N-substituted pyrroles via a Paal–Knorr condensation process. The heterogeneous cobalt–nitrogen catalyst (Co–Nx/C-800-AT) was found to be active for this reaction with formic acid. Formic acid served as a hydrogen donor for the transfer hydrogenation, and also acted as an acid catalyst. More importantly, this method was tolerant of other functional groups, and hence various N-substituted pyrroles were produced with good to excellent yields. The Co–Nx/C-800-AT catalyst was highly stable, and could be reused several times without loss of its catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...