p-TsOH-Catalyzed one-pot transformation of di- and trihydroxy steroids towards diverse A/B-ring oxo-functionalization†
Abstract
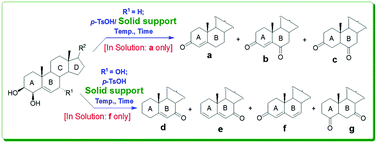
A solid support-mediated p-TsOH-catalyzed milder transformative protocol was developed to furnish diverse ring-A and/or ring-B oxo-functionalized steroids. To furnish interesting isomers involving the A/B-ring of biomolecules in a one-pot approach, only solid supports (and not solution!) were found to be effective. p-TsOH/SiO2-oxidation of 4β-hydroxycholesterol, the major oxysterol in human circulation, into a mixture of cholest-4-en-3-one, cholest-4-ene-3,6-dione, and 5α-cholestane-3,6-dione was the starting point for the investigations herein. The reaction protocol was optimized in detail, and efforts were carried out toward gaining an understanding of the mechanistic aspects favoring the solid support, and a possible synergetic catalytic system involving p-TsOH and SiO2 was expected to be a key part. Application of the novel methodology to 4β,7α-dihydroxy steroids resulted in the desired diverse ketosteroids through oxidation/oxidative dehydration, which generalized the process as a facile multi-oxo-functionalization steroidal transformation.


 Please wait while we load your content...
Please wait while we load your content...