Substituent effect on the N-oxidation of 1,10-phenanthroline derivatives by peroxomonosulfate ion†
Abstract
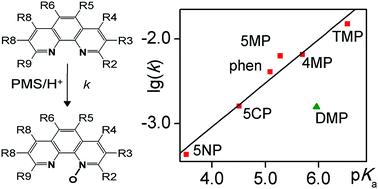
The kinetics of the oxidation of various 1,10-phenanthroline derivatives by peroxomonosulfate ion was studied in detail. The results confirmed that all of these reactions are second order, i.e. first order with respect to each reactant. The large negative activation entropies (ΔS‡ ∼ −130 J mol−1 K−1) imply that these reactions proceed via oxygen atom transfer. Under acidic conditions, the protonation of the mono-N-oxides leads to the formation of an internal hydrogen bond which prevents the oxidation of the second nitrogen atom, i.e. the formation of di-N-oxides. Apart from the kinetic studies, the acid dissociation constants of the studied phen derivatives were also determined by pH-potentiometric titration or combined pH-potentiometry and spectrophotometry. The correlation between the pKa-s of the phenanthroline derivatives and the logarithm of the rate constant of the mono-N-oxidation reaction is linear with the exception of 2,9-dimethyl-1,10-phenanthroline (DMP). Such a correlation strongly suggests that the protonation and mono-N-oxidation of the substrates are governed by the electronic effects of the substituents. In the case of DMP, the significantly slower than expected reaction rate indicates that steric effects may also play an important role in the N-oxidation reactions.


 Please wait while we load your content...
Please wait while we load your content...