A new turn-on benzimidazole-based greenish-yellow fluorescent sensor for Zn2+ ions at biological pH applicable in cell imaging†
Abstract
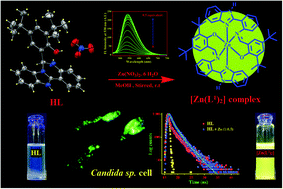
A newly designed and structurally characterized benzimidazole containing compound, 2,4-di-tert-butyl-6-(5,6-dihydro benzo[4,5] imidazo [1,2-c] quinazolin-6-yl)-phenol) (HL), behaves as a ‘turn-on’ fluorescent sensor selective for Zn2+ ions at as low as 39.91 nM within a very short responsive time (15–20 s) in 5 mM HEPES buffer (DMSO/water: 1/5, v/v) at biological pH. Thorough experimental and theoretical (DFT) studies indicate the occurrence of greenish yellow fluorescence through a chelation enhanced fluorescence (CHEF) process. Using this organic moiety (HL) as a probe, almost no interference of other competitive ions in the detection of Zn2+ ions was observed. The reaction of HL with Zn2+ led to the in situ formation of a tridentate monobasic ligand (HL1) to produce the complex as [Zn(L1)2] through a [1,5] sigmatropic-type shift of HL prior to metal coordination. HL is also capable of detecting the intercellular distribution of Zn2+ ions in Candida sp. cells under a fluorescence microscope by developing the image.


 Please wait while we load your content...
Please wait while we load your content...