The role of Lewis acid–base pair sites in ZnO–ZnCr2O4 catalysts for cyclization via dehydrogenative condensation of crude glycerol and 1,2-propanediamine for the synthesis of 2,6-dimethylpyrazine†
Abstract
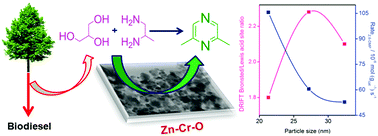
Nano-crystalline mixed oxides of ZnO–ZnCr2O4 (ZC) derived from Zn–Cr–HT precursors were examined for the vapor phase dehydrogenative condensation of crude glycerol and 1,2-propanediamine (1,2-PDA) to synthesize 2,6-dimethylpyrazine (1,2-DMP). The nature of the surface active sites is illustrated by BET-SA, XRD, ESR, H2-TPR, TPD of NH3, TEM, XPS, and pyridine and HCOOH adsorbed DRIFT spectroscopy. The role of acid–base sites in the product distribution is discussed using the catalytic activity data under a kinetic regime. The in situ IR studies revealed that the dehydration of glycerol occurs on weak Lewis acid sites and dehydrogenation takes place on strong basic sites on the catalyst surface. A relationship between surface acid–base strength and the 2,6-DMP rate is established.


 Please wait while we load your content...
Please wait while we load your content...