Highly conductive energy efficient electroless anchored silver nanoparticles on MWCNTs as a supercapacitive electrode†
Abstract
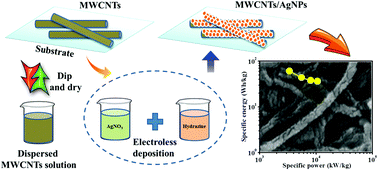
Simple, cost-effective, and room-temperature electroless reduction process has been advanced to anchor silver nanoparticles over multi-walled carbon nanotubes (MWCNTs). The impact of conductive MWCNT/Ag films with respect to structural, morphological, and electrochemical assets has been investigated. The highly stable and large surface area-aligned MWCNTs combined with conductive silver nanoparticles boost the electrochemical performance of the composite electrode. Remarkable specific capacitance of 757 F g−1 along with a significant cyclic stability of 83% over 3000 cycles has been achieved due to the strong synergistic effect between MWCNTs and silver nanoparticles. Moreover, the electrode exhibits a maximum specific energy and power of 60.7 W h kg−1 and 3.3 kW kg−1, respectively. This superior finding obviously offers future pathways for development in the energy storage field.


 Please wait while we load your content...
Please wait while we load your content...