The effect of lithium content on the structure, morphology and electrochemical performance of Li-rich cathode materials Li1+x(Ni1/6Co1/6Mn4/6)1−xO2
Abstract
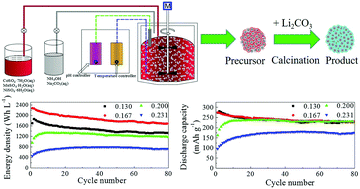
Spherical Li-rich cathode materials Li1+x(Ni1/6Co1/6Mn4/6)1−xO2 (x = 0.130, 0.167, 0.200, 0.231) are synthesized via co-precipitation and solid state reaction, and the effect of lithium content on their structure, morphology and electrochemical performance is investigated. XRD shows that the more lithium content, the more superlattice the structure of the monoclinic Li2MnO3-like component. Besides, as the lithium content increases, the spherical morphology changes little, but the primary particle size increases and the secondary particle surface becomes rough. Among these Li-rich cathode materials, Li1+x(Ni1/6Co1/6Mn4/6)1−xO2 (x = 0.167) displays the highest tap density (over 2.3 g cm−3) and the best electrochemical performance (an initial discharge capacity of 274.1 mA h g−1 in the voltage range of 2.0–4.8 V at 0.1C, a capacity retention of 83.1% after 80 cycles and the highest initial volumetric energy densities of 2277 W h L−1). This is possibly associated with the dense morphology and low cation disordering of Li and Mn in the transition metal layers, which is closely related to the lithium content. This research is expected to definitely contribute to improving the electrochemical performance of Li-rich layered electrodes and making Li-rich materials viable for practical applications in advanced Li-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...