Hydrogenation of 3-hydroxypropanal to 1,3-propanediol over a Cu–V/Ni/SiO2 catalyst†
Abstract
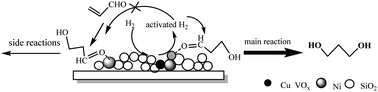
A Ni-based catalyst modified with copper and vanadium (5Cu–20V/30Ni/SiO2) was synthesized and employed in the hydrogenation of 3-hydroxypropanal (3-HPA) to 1,3-propanediol (1,3-PDO). Catalysts were systematically characterized via XRD, TEM, HRTEM, SAED, H2-TPD, N2 physisorption, H2-TPR, and XPS. It was indicated that the addition of Cu and V not only promoted the reduction of Ni2+ species and the generation of active hydrogen species, but also increased the dispersion of Ni species and the interaction between Ni particles and SiO2. The catalytic performance evaluation showed that the (5Cu–20V/30Ni/SiO2) could provide the largest yield of 1,3-PDO (above 76.8%) and highest TOF (4.32 × 103 s−1). The structure–activity relationship was clarified according to the characterization results. The optimized reaction conditions over the 5Cu–20V/30Ni/SiO2 catalyst were obtained as the reaction temperature of 80 °C, a H2 pressure of 2.0 MPa (H2), and an LHSV of 0.4 h−1. The employment of non-noble metals, the relatively low pressure of H2, and the relatively high yield of 1,3-PDO make 5Cu–20V/30Ni/SiO2 an efficient and economic catalyst that may have potential applications in industry.


 Please wait while we load your content...
Please wait while we load your content...