Integration of oxidative arylation with sulfonyl migration: one-pot tandem synthesis of densely functionalized (NH)-pyrroles†
Abstract
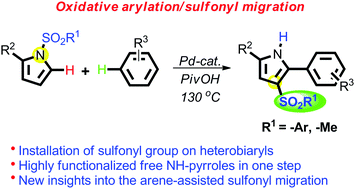
A one-pot synthesis of 2-aryl-3-alkyl/aryl-sulfonyl-(NH)-pyrroles from N-sulfonylpyrroles, developed for the first time, via palladium-catalyzed oxidative C-2 arylation followed by sulfonyl migration is described. The simple, easy access to the highly functionalized free-NH pyrroles secures opportunities for the preparation of compounds with promising biological activities in contemporary organic synthesis. The event of sulfonyl migration from pyrrole-N to C-3 is thermodynamically favored as revealed by density functional methods. The different plausible mechanisms for the migration of the sulfonyl group are also discussed.


 Please wait while we load your content...
Please wait while we load your content...