Synthesis of novel profluorescent nitroxides as dual luminescent-paramagnetic active probes†
Abstract
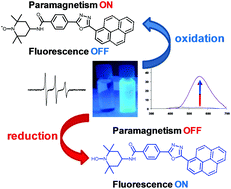
We describe herein the synthesis of new profluorescent nitroxides based on 2,5-disubstituted-1,3,4-oxadiazoles as fluorescent moieties and the 2,2,6,6-tetramethylpiperidine-N-oxyl radical (TEMPO) as paramagnetic probes. The synthesis and optical and electronic properties of the oxadiazole-based precursors of the profluorescent nitroxides, as well as the nitroxides and their reduced forms are also described. Physical investigations (i.e. absorption and emission spectroscopy, cyclic voltammetry) indicate a different luminescence behaviour function to the oxadiazole substituent. A linear response to reducing agents, i.e. sodium ascorbate, monitored by fluorescence spectroscopy, suggests the possibility of using the synthesized compounds as potent active probes in detection of various analytes of interest.


 Please wait while we load your content...
Please wait while we load your content...